- Development of the monitoring concept
- Risk Assessment via Risk Management Plan (RMP)
- Coordination of monitoring activities
- Training of the CRA team
- Selection of sites
- Conducting training at the sites
- "Hotline" for inquiries from sites
- Review of monitoring reports
- Conducting regular team meetings

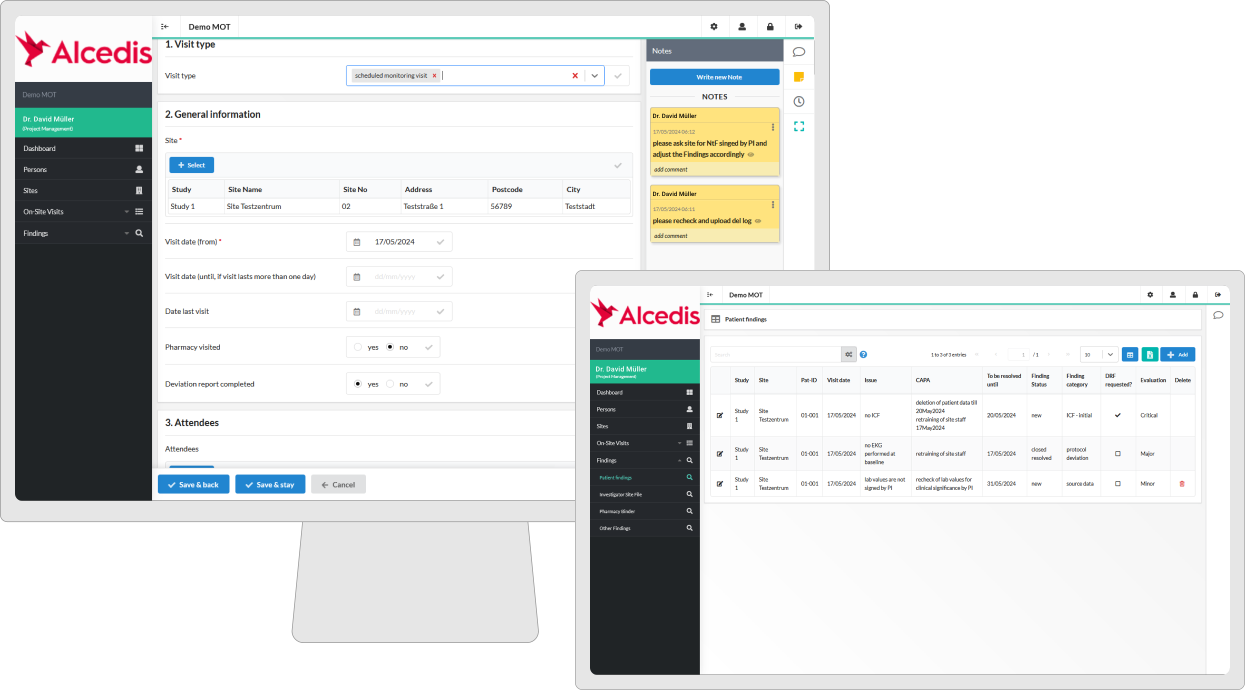
Monitoring Software
Unlock the power of seamless monitoring with our stand-alone monitoring software, ensuring GCP-compliant documentation and meticulous site management.
- Optimized Visit Planning: Effortless management of sites, staff, and CRAs through streamlined visit planning with automated tracking of visit progress.
- Efficient Tooling: Streamline review procedures with digital signatures, instant follow-up letter dispatch, and document handling.
- Overview and Reporting: Optimized sponsor oversight with follow-up of findings, deviations, and serious breaches, complemented by site-specific risk assessments based on findings.
Risk-based Monitoring
We optimize clinical trial monitoring with our risk-based strategy to prioritize the critical areas of each study, ensuring the utmost efficiency and effectiveness in oversight.
- Comprehensive Risk Analysis: We conduct a risk analysis for every clinical trial protocol, meticulously examining potential risks to both patient safety and data integrity.
- Continuous Risk Evaluation: Our process entails ongoing evaluation of identified risks, supported by regular risk reviews that guarantee proactive risk management throughout the clinical trial duration.
- Strategic Risk Control: Leveraging our risk evaluation findings, we strategize risk prevention, mitigation, or acceptance measures. High-risk areas are targeted for specialized control strategies, enhancing overall trial safety and data quality.

Latest Whitepaper
Our summarization of EUCROF's propositions on leveraging remote source data verification and reviews (rSDV/rSDR) in clinical trials. Gain insights and recommendations for seamless integration, ensuring data consistency and participant privacy while reducing site burden.
Download
Let's create an impact. Together.
Create a powerful environment for research and contact us today.
Contact us!




