Alcedis Plugins
Plug-in the final finish with readily available functionalities for the most common and important processes developed by best practice.
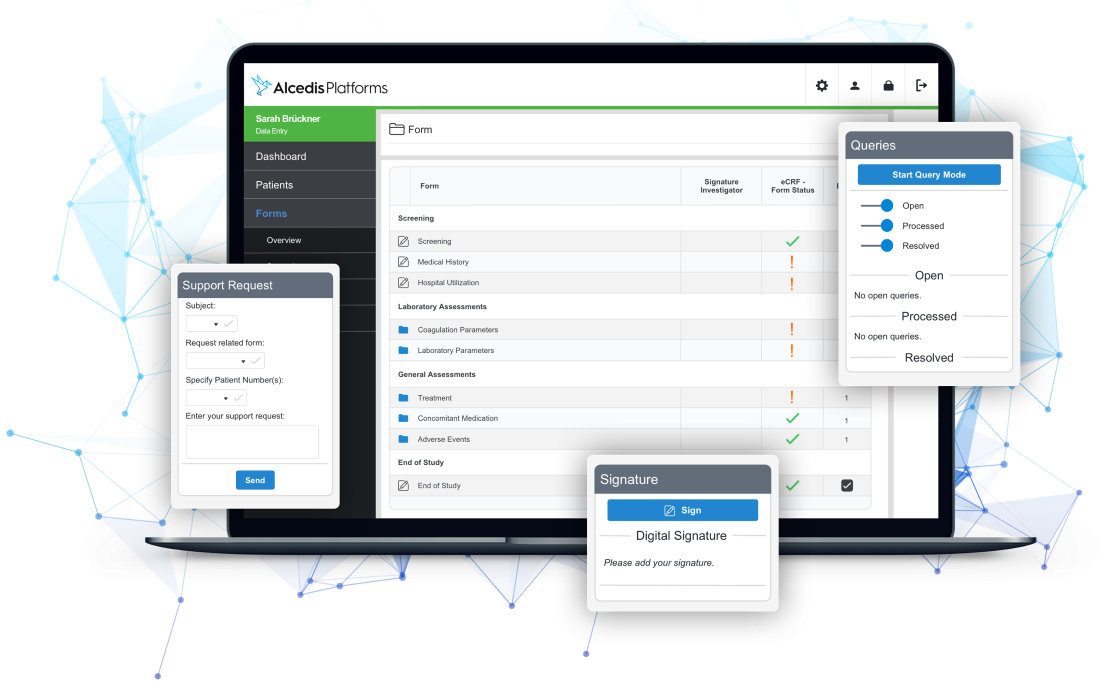
Simplify Documentation
With smart and automated real-time data validation, we efficiently reduce up to 80 % of manual data cleaning efforts. Patient data is easily monitorable in modern and lucid interfaces for power users like CRAs, data- or project managers. Role-based access control and audit trails grant full transparency.
- Real-time data validation
- Easily monitorable data

Streamline Processes
User adaptive dashboard instruments are providing valuable oversight information. Status of site activities, patient recruitment, completeness of documentation, reached milestones and much more can be individually bookmarked or dynamically found via a semantic, elastic search. Forecasting systems and alerts ensure that the study is on track.
- In-App Bookmarking to keep the most relevant information always in sight
- Elastic search to find answers to important questions quickly

Launch your Alcedis Platform™!
Let us create a high-performance environment for science that matters. Together!
Contact us


























